
NEWS
Why do metals always "glow" and what is the principle behind their luster?
Have you ever wondered why metals are always so shiny, whether it's gold, silver or copper, they all have a
unique sheen? Today, we will explore this question together and uncover the scientific mystery behind the
metallic luster!
It's a physics problem, it's a chemistry problem, but ultimately it's a physics problem.
In fact, metallic luster is a strong specular reflection, as long as a large amount of incoming light can be
concentrated back, will produce metallic luster, oil stains on water and even insect exoskeletons have this
ability. However, compared with other materials, the specular reflection ability of metal is particularly worth
mentioning.
Specular reflection is probably the simplest concept in middle school physics, and our understanding of it
often focuses on the point that the reflective surface is very smooth. However, simply think about it, not all
materials can shine like metal after polishing, so there must be other reasons for metal to shine so.

Why do metals always "glow" and what is the principle behind their luster?
This is the elytra, breast plate and shield of a gilding worm. Beetle exoskeletons are often extremely smooth,
creating a metallic texture
1. Basic structure of metal
To understand why metals shine, we first need to understand the basic structure of metals. Metals are made
up of tightly packed atoms, and the connections between these atoms are very strong. This connection
prevents the metal atoms from moving freely between each other, forming what is called a "metallic bond."
When we were in middle school, we learned that metal elements have fewer outer electrons and are easy to
"lose", showing strong reducibility. These "lost" electrons form a sea of free electrons inside the metal crystal.
A metal crystal is just such a diffuse plasma.
2. Luster of free electrons and metals
The luster of a metal is mainly due to the free electrons within it. When the electromagnetic field reaches the
metal surface, it forms "ripples" on the surface of the ocean made up of free electrons in the metal crystal,
that is, the electromagnetic wave causes the plasma to oscillate.
Plasma oscillations caused by high-frequency electromagnetic waves have a natural frequency, called the
"plasma frequency", which is related to the density of free electrons in the plasma, and is independent of the
intensity of the electromagnetic wave. What followed, however, was very different from the macro world.
In the macroscopic world, no matter how much force can change the motion of the object, or no matter how
much force can make the oscillator vibrate. But in the microscopic world, quantum effects manifest themselves:
light is an electromagnetic wave, but also a particle, and the energy of a photon is determined by its frequency.
When a photon enters the metal's sea of free electrons, it must expend energy to excite a plasma oscillation of
the plasma frequency before the rest can continue to propagate. And if a photon is not energetic enough to
trigger such an oscillation, it will bounce off unchanged, no matter how long it is illuminated, unlike the macro
world, which can accumulate.

Why do metals always "glow" and what is the principle behind their luster?
In other words, only if the frequency of a photon is greater than a certain value, its energy can exceed the
absorption frequency of the plasma in order to be absorbed, otherwise it will be reflected away. Plasma frequencies
in metals, however, are quite high, often reaching the ultraviolet range. So lower frequency visible light is strongly
reflected, which is what we see as metallic luster.
3. Different metals, different gloss
Although all metals can reflect light, the reflected light of different metals is different, and some metals can even
absorb some special bands of visible light, thus showing a unique color. This is due to some complex physical
mechanisms.
For example, the gold melancholic relativistic effect can absorb blue-violet light, showing a bright yellow; Copper's
electrons can transition between the two subelectron orbitals, which can absorb blue to yellow-green light and
appear red, while being darker than other metals.
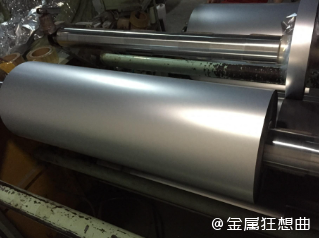
The surface of the metal is usually very smooth, which is also an important reason for its luster. The smooth surface
means that light is not scattered when reflected, resulting in a uniform shine. Objects with rough surfaces, such as
wood or stone, scatter light in multiple directions, so they don't have the same luster as metal.
Why do metals always "glow" and what is the principle behind their luster?
4. Ferrous metal is not black
I don't know if you have noticed, when it comes to metal, we never seem to associate it with black, but "black metal"
is indeed a major category of metals!
The word "black" can easily make people mistakenly think that black metal must be black, and non-ferrous metal
must be colorful. Based on this, many people think that silver-white iron, chromium and silver-gray manganese should
not be "black" metals. So why do they and their alloys belong to the category of ferrous metals?
The reason is this: Ferrous metals are the industrial collective name for iron, chromium and manganese, and also include
alloys of these three metals. In fact, pure iron and chromium are silver-white, while manganese is silver-gray. Because the
surface of steel is usually covered with a layer of black ferric oxide, and manganese and chromium are mainly used in the
smelting of black alloy steel, so it is mistaken for "black" metals.
The English translation of "Nonferrous Metals" is "Nonferrous Metals", which corresponds to the English translation of
"Ferrous Metals". According to an old gentleman who has been working in the non-ferrous metal industry for a long time,
the process of translating "Ferrous Metals" and "Nonferrous Metals" into "ferrous metals" and "nonferrous metals" is also
an interesting story.
The earliest translation of "Ferrous Metals" and "Nonferrous Metals" scholars believe that if it is directly translated into
"ferrous metals" and "non-ferrous metals", or "ferrous metals" and "non-ferrous metals", although considering the accuracy
of the translation, it is not concise enough, and it is also very difficult to use. Therefore, the scholar jumped out of the
conventional thinking, starting from the optical properties of metals, and carried out a re-creation in translation.
Pure iron is silver white, but the iron often seen in People's Daily work and life is black. This is because the surface of iron is
covered with a black oxide film: iron tetroxide. It is also because of this layer of oxide film that iron leaves a black impression
on people, so from this point of view, the "Ferrous Metals" is creatively translated as "black metal".
With the presence of "ferrous Metals", and considering that silver is white, gold is gold, copper is yellow, and lead is gray,
"non-ferrous metals" are presented with colorful optical properties, so "Nonferrous Metals" is translated into "nonferrous
metals". Since then, "Ferrous Metals" and "Nonferrous Metals" have become the Chinese "Ferrous metals" and "Nonferrous
metals" in the industry to spread.
Source: Guo Ge talk science, popular science China, TANJA precision TANja
Note: All pictures in the article are network reprint pictures, infringement is deleted!






